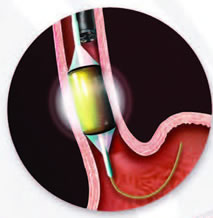
While you are sedated, a device is inserted through the mouth into the esophagus and used to deliver a controlled level of energy and power to remove a thin layer of diseased tissue. Less than one second of energy removes tissue to a depth of about one millimeter. The ability to provide a controlled amount of heat to diseased tissue is one mechanism by which this therapy has a lower rate of complications than other forms of ablation therapy.
Larger areas of Barrett’s tissue are treated with the balloon-mounted catheter. Smaller areas are treated with the endoscope-mounted catheter. Both are introduced during an upper endoscopy procedure, which is a thin, flexible tube inserted through a patient’s mouth.
Radiofrequency ablation for Barrett’s esophagus has been used in more than 60,000 cases and the devices are cleared by the U.S. Food and Drug Administration. The balloon-based catheter has been available commercially since January 2005, the endoscope-mounted catheter since January 2007.
A clinical trial by Fleischer, et al. showed that 98.4% of people were free of Barrett’s at a follow-up exam 30 months after two or three RFA treatments. Studies show that when the Barrett’s tissue is removed, it is typically replaced by normal, healthy tissue within three to four weeks. Recent five year follow-up of longer term trials shows that the effects of radiofrequency ablation are durable.